I. Introduction to Ptosis
Definition and Anatomical Basis of Eyelid Elevation
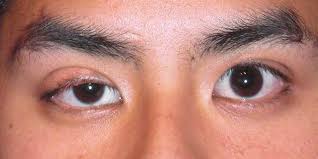
Ptosis, clinically referred to as blepharoptosis, denotes the abnormally low positioning or drooping of the upper eyelid. This condition can significantly diminish or entirely obstruct vision, alongside presenting notable cosmetic concerns . The term “ptosis” originates from the Greek word for “falling,” specifically describing the drooping of a body part, with “blepharoptosis” precisely indicating upper eyelid drooping when the eyes are in the primary gaze position .
The intricate process of eyelid elevation is primarily governed by two key muscles: the levator palpebrae superioris muscle (LPS) and Müller’s muscle, also known as the superior tarsal muscle . The LPS muscle originates from the lesser wing of the sphenoid bone, extending anteriorly over the superior rectus muscle. It disperses into multiple insertions, attaching anteriorly into the upper eyelid skin, inferiorly onto the anterior surface of the upper tarsal plate, and to the superior conjunctival fornix . The LPS receives its primary innervation from the oculomotor nerve (cranial nerve III) . Müller’s muscle, a sympathetically innervated smooth muscle, contributes approximately 2 millimeters of upper eyelid lift . Additionally, the frontalis muscle, controlled by cranial nerve VII (the facial nerve), provides a minor contribution to eyelid movement and can act as a compensatory mechanism .
The distinct innervation and roles of the levator palpebrae superioris and Müller’s muscle underscore that ptosis can stem from various points within this complex system, including the muscle itself, the neuromuscular junction, or the specific nerve pathways controlling them. The involvement of the frontalis muscle as a compensatory mechanism further highlights the intricate interplay of muscles and nerves in maintaining the eyelid’s position. This integrated system implies that a thorough understanding of ptosis extends beyond merely identifying the drooping eyelid; it necessitates pinpointing the exact anatomical and neurological component affected to guide precise and effective treatment. Therefore, ptosis is not simply a cosmetic issue but a complex neuromuscular disorder involving multiple anatomical structures and their precise innervation, making accurate diagnosis critical for targeted therapeutic strategies.
Clinical Significance and Impact on Vision and Cosmesis
Ptosis can manifest as a significant functional deficit, directly obstructing vision, or as a prominent cosmetic concern that impacts a patient’s identity and self-perception. Visual obscuration due to drooping eyelids is a frequently reported complaint among affected individuals .
If left unaddressed, particularly in pediatric patients, severe ptosis can lead to profound and irreversible complications. One of the most critical is deprivation amblyopia, commonly known as “lazy eye,” which occurs when the drooping eyelid physically blocks the visual axis, causing the brain to favor the eye with clearer vision. This can result in permanent vision loss in the affected eye if not treated during the critical period of visual development, typically by age six . Another ophthalmological complication is astigmatism, where the pressure exerted by the eyelid on the cornea can alter the eye’s shape, leading to distorted or wavy vision . Children with ptosis often adopt compensatory head postures, such as tilting their head backward (chin-up position) or elevating their eyebrows, in an attempt to see beneath the drooping eyelid . While these actions temporarily improve vision, prolonged adoption of such postures can lead to chronic head and neck problems, including muscle strain and skeletal deformities over time . Adults may experience a persistent feeling of heaviness in the eyes, visual obstruction, and significant cosmetic complaints that can affect their quality of life .
The consistent emphasis on both the functional (vision obstruction, amblyopia risk) and cosmetic (appearance, self-perception) impacts of ptosis highlights a dual burden for affected individuals. This dual challenge dictates that treatment decisions must consider both aspects. For children, the functional impact, particularly the risk of amblyopia, takes precedence due to the critical period of visual development, necessitating early and often urgent intervention . For adults, while visual impairment remains a primary concern, cosmetic considerations frequently drive the desire for treatment, emphasizing the psychosocial dimension of the condition. Therefore, ptosis management extends beyond mere physical correction; it involves addressing both critical visual development in children and significant quality-of-life impacts, encompassing functional and aesthetic dimensions, across all age groups.
II. Etiology and Classification of Ptosis
Ptosis is broadly categorized into two main types based on its onset: congenital, meaning present at birth, or acquired, developing later in life. Each of these categories encompasses further subcategories defined by their specific underlying causes .
Congenital Ptosis: Causes and Characteristics
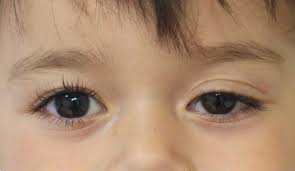
Congenital ptosis is characterized by the presence of a droopy eyelid or eyelids from birth or within the first year of life . It represents the most common type of ptosis overall and exhibits a higher prevalence in males .
The primary cause of congenital ptosis is typically a localized myogenic dysgenesis, which refers to the maldevelopment of the levator palpebrae superioris muscle . In this condition, normal muscle fibers are replaced by fibrous and adipose tissues, which significantly diminishes the muscle’s ability to contract and relax effectively . This structural abnormality leads to reduced levator function and frequently manifests as an absent or weak upper lid crease, a key clinical indicator . During downgaze, the ptotic eyelid may appear paradoxically higher due to the levator muscle’s inability to relax sufficiently .
Less common etiologies of congenital ptosis include:
- Simple congenital ptosis: This is idiopathic in origin .
- Congenital ptosis with superior rectus muscle weakness: Often termed double-elevator palsy .
- Marcus Gunn jaw-winking ptosis: A congenital synkinetic ptosis where the motor innervation of the external pterygoid muscle is misdirected to supply the ipsilateral levator muscle, resulting in eyelid elevation during mastication .
- Third cranial nerve palsy: Can be present congenitally .
- Horner syndrome: Characterized by mild ptosis, pupillary miosis (constriction), anhidrosis (lack of sweating), and ipsilateral iris heterochromia (different colored irises) .
- Secondary to birth trauma .
- Periorbital tumors: Such as plexiform neurofibromatosis, neuroblastoma, lymphoma, rhabdomyosarcoma, or neuroma leukemias, which can induce mechanical ptosis .
- Pseudotumor of the orbit: Ptosis induced by inflammatory disease of the orbit secondarily affecting the eyelids .
The consistent emphasis on “myogenic dysgenesis” and the replacement of normal muscle fibers with fibrous and adipose tissue in congenital ptosis points to a fundamental developmental anomaly rather than an acquired injury or disease. This developmental basis explains why surgical interventions in children often focus on strengthening or replacing the poorly functioning muscle, and why the long-term outlook may differ from acquired forms. The characteristic absent or weak lid crease in congenital ptosis is a direct clinical manifestation of this underlying developmental issue, as the normal anatomical attachments crucial for crease formation are compromised. Therefore, congenital ptosis is primarily a developmental muscular disorder, distinct from acquired forms, which influences its clinical presentation and necessitates early intervention to prevent vision development issues.
Acquired Ptosis
Acquired ptosis develops later in life and results from a variety of factors, including the natural aging process, trauma, muscle weakness or stretching, or nerve damage . Among all acquired cases, aponeurotic ptosis is the most common type, typically presenting in late adulthood .
Aponeurotic (Involutional) Ptosis: Causes and Features
Aponeurotic ptosis is the most prevalent form of adult ptosis, commonly appearing in the fifth or sixth decade of life . Its primary etiology is a defective levator aponeurosis, which can occur due to aging, trauma, or as a postoperative complication . The underlying pathogenesis involves the dehiscence or disinsertion of the levator aponeurosis, or its stretching and thinning, often a result of decreasing tone and thinning of the levator muscle with age . Prolonged use of contact lenses has also been identified as a contributing factor .
Characteristic clinical features of aponeurotic ptosis include preserved good levator function, a high lid crease, a thin upper eyelid with redundant skin, and the affected eyelid appearing lower on downgaze . The margin crease distance (MCD) is typically higher than normal in these patients .
The strong association of aponeurotic ptosis with aging, often referred to as “involutional” ptosis, and the specific mechanism of levator aponeurosis dehiscence or stretching, highlight a common degenerative pathway. This distinguishes aponeurotic ptosis from congenital or neurogenic causes, where the primary pathology is developmental or neurological, respectively. The preservation of good levator function despite the drooping is a key diagnostic feature, indicating that the muscle itself remains functional, but its connection to the eyelid is compromised. This understanding is crucial because it directly influences the choice of surgical repair, focusing on reattaching or tightening the aponeurosis rather than addressing muscle or nerve issues. Therefore, aponeurotic ptosis is predominantly a consequence of age-related wear and tear on the levator aponeurosis, distinct from other etiologies by its preserved levator muscle function and characteristic clinical signs like a high lid crease.
Neurogenic Ptosis: Specific Conditions
Neurogenic ptosis arises from defective innervation of either the levator muscle or Müller’s muscle . This condition stems from problems within the nerve pathways that control eyelid muscles, specifically dysfunction in the oculomotor nerve (cranial nerve III) or the sympathetic nerve supply .
Common conditions leading to neurogenic ptosis include:
- Third Cranial Nerve (Oculomotor Nerve) Palsy: This results from lesions along the oculomotor nerve. Pupil-involving third nerve palsy is frequently caused by a posterior communicating artery aneurysm compressing the nerve, constituting a medical emergency . Pupil-sparing third nerve palsy is typically due to an ischemic vascular cause and often resolves spontaneously within three months . Other causes include inflammation, trauma, or tumors affecting the nerve’s course . Clinically, it presents with ptosis and restricted movements of adduction, elevation, and depression of the eyeball . Pupillary involvement, manifesting as mydriasis (dilation), may or may not be present . Bell’s phenomenon, the upward and outward rotation of the eye during eyelid closure, is usually poor in these cases .
- Horner Syndrome (Oculosympathetic Paresis): This condition occurs due to the interruption of the sympathetic nerve supply to Müller’s muscle and the dilator pupillae muscle . It is characterized by a classic triad of mild ptosis, pupillary miosis (constriction), and anhidrosis (lack of sweating on the affected side of the face) . An apparent enophthalmos (sunken eye) may also be observed . Pupillary anisocoria (unequal pupil size) is more pronounced in dim illumination . In cases where Horner syndrome develops during childhood, iris heterochromia (different colored irises) may occur due to decreased melanin production, which is controlled by the sympathetic pathway .
- Marcus Gunn Jaw-Winking Syndrome: This is a congenital synkinetic ptosis where the motor innervation of the external pterygoid muscle is misdirected to supply the ipsilateral levator muscle, causing the eyelid to elevate with mastication .
- Other Causes: Neurogenic ptosis can also be caused by conditions such as multiple sclerosis, diabetic neuropathy, head trauma, brain tumors, and cerebrovascular accidents (stroke) .
The detailed causes of neurogenic ptosis, particularly third nerve palsy and Horner syndrome, are not merely isolated ocular conditions but frequently serve as indicators of serious underlying systemic or neurological pathologies, such as intracranial aneurysms, stroke, tumors, or multiple sclerosis . The sudden onset of third nerve palsy, for instance, is explicitly recognized as a “medical emergency” due to the potential presence of a brain aneurysm . This implies that a diagnosis of neurogenic ptosis necessitates a rapid and thorough systemic neurological workup, extending beyond a sole ophthalmological assessment, to identify and treat potentially life-threatening conditions. The characteristic triad of Horner syndrome (ptosis, miosis, anhidrosis) acts as a critical diagnostic clue for sympathetic pathway disruption. Therefore, neurogenic ptosis, especially with acute onset or specific associated signs like pupillary changes and eye movement limitations, functions as a critical “red flag” for potentially severe and life-threatening systemic neurological conditions, demanding immediate and comprehensive medical evaluation.
Myogenic Ptosis: Specific Conditions
Myogenic ptosis is caused by a primary myopathy of the levator muscle or a defect at its neuromuscular junction . This condition involves a direct weakening of the levator palpebrae superioris muscle or its tendon .
Common conditions leading to myogenic ptosis include:
- Myasthenia Gravis (MG): An autoimmune disorder characterized by impaired communication between nerves and muscles, leading to muscle weakness and fatigue . Ptosis is a frequent symptom, often pupil-sparing, and may exhibit diurnal variability (worsening throughout the day) or increase with fatigue . MG is also known to correlate with thyroid disorders, necessitating assessment of thyroid function when suspected .
- Ocular Myopathy: This involves a direct affection and dysfunction of the levator muscle itself .
- Simple Congenital Ptosis: This form of ptosis is caused by levator muscle dystrophy .
- Blepharophimosis Syndrome: A genetic disorder listed as a cause of myogenic ptosis .
- Other Systemic Disorders: Myogenic ptosis can also be a manifestation of other systemic conditions, including myotonic dystrophy, oculopharyngeal muscular dystrophy (OPMD), chronic progressive external ophthalmoplegia (CPEO), facioscapulohumeral muscular dystrophy, congenital myopathies, and mitochondriopathy .
Myogenic ptosis, particularly Myasthenia Gravis, is presented not merely as an isolated eyelid issue but often as a localized manifestation of a broader systemic autoimmune or genetic disorder . The diagnostic tests employed for MG, such as the Edrophonium test, Ice test, and specific antibody testing, are designed to identify this systemic connection . This emphasizes that effective management of the ptosis often requires treating the underlying systemic disease rather than focusing solely on the eyelid. This highlights the importance of a comprehensive medical workup that extends beyond the ocular examination to ensure appropriate overall patient management.
Mechanical Ptosis: Causes
Mechanical ptosis occurs when the function of the levator muscle is impaired due to the mass effect of an abnormal external structure physically weighing down the eyelid .
Examples of such structures include:
- Neoplasms (tumors) .
- Chalazion: A common cyst of the eyelid .
- Contact lenses: Particularly those lodged in the upper fornix .
- Scarring: Resulting from previous trauma or inflammation .
Unlike neurogenic or myogenic ptosis, mechanical ptosis is characterized by a direct physical obstruction or weight on the eyelid. This implies that the levator muscle and its innervation may be entirely healthy, but their function is physically impeded. This distinction is crucial for treatment planning, as removing the offending mass is often the primary and most effective intervention, rather than complex muscle or nerve repair procedures. Therefore, mechanical ptosis represents a direct physical impediment to eyelid elevation, meaning treatment primarily focuses on removing the external mass rather than addressing intrinsic muscle or nerve dysfunction.
Traumatic Ptosis: Causes and Subcategories
Traumatic ptosis results from any direct or indirect trauma to the eyelid .
The mechanisms leading to traumatic ptosis can include:
- Levator transection .
- Cicatrization (scarring) .
- Eyelid laceration .
- Orbital rooftop fracture with ischemia .
Traumatic ptosis is a heterogeneous condition, and its cases can be further classified based on the primary mechanism of injury into several subcategories :
- Traumatic myogenic ptosis: Occurs due to direct injury to the levator muscle and/or Müller’s muscle, such as a laceration caused by broken glass .
- Traumatic aponeurotic ptosis: Results from dehiscence or disinsertion of the levator aponeurosis from its tarsal insertion, without significant injury to the muscle belly. Examples include vigorous lid pulling or stretching due to a large hematoma .
- Traumatic mechanical ptosis: Caused by cicatricial bands tethering, stiffening, or weighing down the lid, thereby limiting its opening .
- Traumatic neurogenic ptosis: Occurs as a result of neural injury to cranial nerve III or sympathetic fibers, affecting any point along their course .
- Traumatic mixed mechanism ptosis: A combination of one or more injury mechanisms .
The breakdown of traumatic ptosis into these subcategories reveals that “trauma” is not a singular cause but rather a trigger that can lead to various underlying pathophysiologies. This implies that even after an injury, the diagnostic approach must still precisely identify the specific anatomical structure damaged—whether it be the muscle, aponeurosis, nerve, or an external mass—to guide appropriate and effective treatment. It also highlights that ptosis secondary to blunt trauma may resolve spontaneously, suggesting an initial period of observation before surgical intervention is considered . Therefore, traumatic ptosis is a complex category that can manifest through diverse underlying mechanisms (myogenic, neurogenic, aponeurotic, mechanical), requiring precise identification of the damaged structure for effective management, often after an initial observation period.
Pseudoptosis
Pseudoptosis is not true ptosis but an apparent drooping of the eyelid caused by abnormalities in structures other than the levator muscle or its direct innervation .
Common causes of pseudoptosis include:
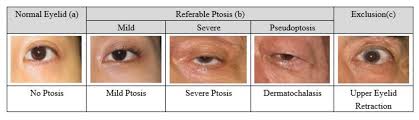
- Dermatochalasis: Excessive or redundant eyelid skin .
- Brow ptosis: Drooping of the eyebrow .
- Hypotropia: Downward deviation of the eye .
- Microphthalmos: An abnormally small eye .
- Anophthalmos: The congenital absence of one or both eyes .
- Phthisis bulbi: A shrunken, non-functional eye .
- Superior sulcus defect: A hollowing in the upper eyelid due to orbital volume loss .
- Contralateral eyelid retraction: Where the opposite eyelid is abnormally elevated, making the affected eyelid appear droopy by comparison .
The concept of pseudoptosis is crucial because it mimics true ptosis but has fundamentally different underlying causes and, consequently, requires entirely different treatment strategies . Misdiagnosing pseudoptosis as true ptosis could lead to inappropriate and ineffective interventions, potentially exacerbating the patient’s condition or failing to address the actual problem. This emphasizes the critical importance of a thorough diagnostic evaluation to differentiate between actual levator muscle dysfunction and other conditions that merely create the appearance of a droopy lid. Therefore, distinguishing true ptosis from pseudoptosis is paramount for accurate diagnosis and effective treatment, as their etiologies and management strategies are fundamentally different.
Table 1: Classification and Key Etiologies of Ptosis
| Classification | Primary Etiology | Key Characteristics | Common Examples/Associated Conditions |
| Congenital | Maldevelopment of levator muscle (myogenic dysgenesis) | Present from birth/early life, reduced levator function, often absent lid crease, lid lag in downgaze | Simple congenital ptosis, Marcus Gunn jaw-winking syndrome, congenital Horner syndrome, Third nerve palsy (congenital) |
| Acquired | Various factors developing later in life | Variable presentation based on type | |
| Aponeurotic (Involutional) | Dehiscence/stretching of levator aponeurosis | Most common adult type, good levator function, high lid crease, thin eyelid, redundant skin | Aging, trauma, ocular surgery, prolonged contact lens use |
| Neurogenic | Defective innervation of levator or Müller’s muscle | Associated with neurological signs (pupil, eye movement) | Third cranial nerve palsy, Horner syndrome, Multiple Sclerosis, Myasthenia Gravis (can also be myogenic) |
| Myogenic | Levator muscle myopathy or neuromuscular junction defect | Muscle weakness, often progressive, may have systemic associations | Myasthenia Gravis, Ocular myopathy, Myotonic dystrophy, Oculopharyngeal muscular dystrophy (OPMD), Chronic progressive external ophthalmoplegia (CPEO) |
| Mechanical | Mass effect of external structure | Physical obstruction of eyelid elevation | Neoplasms, Chalazion, Foreign bodies (e.g., contact lens in fornix), Scarring |
| Traumatic | Direct or indirect injury to eyelid structures | History of trauma, can involve muscle, aponeurosis, nerve, or scarring | Eyelid laceration, orbital fracture, blunt trauma |
| Pseudoptosis | Apparent drooping due to other structural abnormalities | Normal levator function, no true muscle/nerve dysfunction | Dermatochalasis, Brow ptosis, Hypotropy, Anophthalmos, Contralateral eyelid retraction |
This table serves as a valuable tool for clinicians by providing a concise yet comprehensive overview of the diverse classifications and etiologies of ptosis. The organized presentation of information into distinct categories and their subtypes, along with key characteristics and common associated conditions, facilitates differential diagnosis. For instance, the presence of an absent lid crease immediately directs attention towards congenital ptosis, while good levator function coupled with a high lid crease suggests aponeurotic ptosis. This structured approach is fundamental for identifying the specific etiology, which in turn guides the selection of the most appropriate treatment strategy.
III. Clinical Presentation
Common Symptoms and Patient Complaints
Patients presenting with ptosis typically report a range of symptoms, most commonly including a noticeable drooping of one or both eyelids, a persistent feeling of heaviness in the eyes, and visual obscuration . The degree of visual impairment can vary significantly, from mild blurring to complete occlusion of the visual field . Cosmetic concerns are also a frequent complaint, as the drooping eyelid can affect a patient’s appearance and self-perception .
Beyond these primary complaints, other associated symptoms may include excessive rubbing of the eyes, increased tearing, and a general sense of tiredness or achiness around the eyes . A thorough patient history is critical, as the age of onset and duration of symptoms provide crucial information for differentiating between congenital and acquired cases . Clinicians also inquire about aggravating or relieving factors, such as diurnal variability (e.g., worsening of ptosis later in the day, characteristic of Myasthenia Gravis) . Associated symptoms like diplopia (double vision), pain, lid swelling, dysphagia (difficulty swallowing), or generalized muscle weakness can provide important clues to the underlying etiology, guiding further diagnostic steps .
Key Physical Signs and Compensatory Mechanisms
Clinical evaluation of ptosis commences immediately upon the patient’s entry into the examination room, with careful observation of both eyes and the patient’s overall appearance . Patients with significant ptosis often exhibit characteristic compensatory changes in an attempt to improve their vision. These include a backward tilted head, commonly referred to as a “chin-up” position, wrinkling of the forehead skin, and elevated eyebrows . These actions are direct physiological responses to the visual obstruction caused by the drooping eyelid, aiming to lift the eyelid margin away from the pupil. While effective in the short term, prolonged adoption of these compensatory postures can lead to chronic neck problems and tightened forehead muscles over time .
Upon closer inspection, the upper lid margin in ptosis typically covers more than 2 mm of the cornea, resulting in a narrower palpebral fissure than normal . Any scars, swelling, or abnormal structures near the eyelids should be carefully noted, as these can indicate a mechanical or traumatic etiology .
Specific clinical signs are crucial for differential diagnosis:
- Absent upper lid crease: This is a hallmark sign strongly indicative of congenital ptosis .
- Pupillary function abnormalities: These are important indicators of neurogenic causes. Anisocoria (unequal pupil size) with miosis (pupil constriction) suggests Horner syndrome, while mydriasis (pupil dilation) points towards a third cranial nerve palsy .
- Ocular motility issues: Assessment of eye movements is essential to identify cranial nerve III paresis or other extraocular muscle involvement that may accompany ptosis .
- Jaw-winking sign: This specific test is performed to rule out Marcus Gunn jaw-winking syndrome, a congenital synkinetic ptosis .
- Bell’s phenomenon: The upward and outward rotation of the eye during attempted eyelid closure is assessed preoperatively to determine a patient’s suitability for ptosis surgery and to evaluate the risk of postoperative exposure keratopathy (corneal drying) . A poor Bell’s phenomenon may necessitate avoiding or intentionally undercorrecting ptosis surgery to protect the cornea .
- Lagophthalmos and lid lag on downgaze: The presence of incomplete eyelid closure (lagophthalmos) or the upper eyelid lagging behind the globe during downward gaze should be documented, as these conditions can worsen after surgical intervention .
- Brow ptosis or dermatochalasis: Drooping of the eyebrow or excessive eyelid skin should be documented, as blepharoplasty (surgical removal of excess skin) is often combined with ptosis repair in involutional (age-related) cases .
The emphasis on observing the patient’s general appearance, compensatory postures, and specific eyelid and ocular signs before detailed measurements highlights that clinical observation serves as the initial and foundational step in diagnosis. These subtle cues, such as a chin-up position or a wrinkled forehead, are direct physiological responses to visual impairment and can immediately guide the clinician towards the presence and severity of ptosis, even before quantitative measurements are taken. This approach underscores the critical role of skilled clinical examination in ophthalmology, where astute observation can significantly streamline the diagnostic process.
IV. Diagnostic Evaluation
A thorough history and comprehensive clinical examination are of paramount importance in accurately determining the etiology of ptosis and subsequently formulating an appropriate treatment plan .
Comprehensive History Taking
The patient’s history should meticulously cover the age of onset, the progression, and the duration of the ptosis . It is crucial to inquire about any aggravating or relieving factors, such as diurnal variation, which is characteristic of Myasthenia Gravis where symptoms may worsen throughout the day . Documentation of associated symptoms like diplopia (double vision), pain, lid swelling, dysphagia (difficulty swallowing), or generalized muscle weakness can provide invaluable clues to the underlying etiology . Clinicians must also diligently rule out predisposing factors, including prior trauma, ocular or eyelid surgery, prolonged contact lens use, and recent botulinum toxin injections . A detailed family history of ptosis is essential to exclude hereditary disorders . Old photographs can offer significant insight into the precise time of onset, particularly when the patient’s recollection is inconclusive . Furthermore, any systemic illnesses (e.g., diabetes, hypertension, thyroid disorders), mental health issues, and current medication history (e.g., blood thinners like aspirin, which may need to be discontinued preoperatively) require careful documentation .
Detailed Ocular and Eyelid Examination
The clinical examination commences with an initial observation of the patient’s facial symmetry, the presence of frontalis overaction (compensatory eyebrow elevation), and any chin-up or head-tilt postures adopted to improve vision .
A general ocular examination includes assessing visual acuity and refraction, performing a cover test to check for hypotropia and rule out pseudoptosis, evaluating extraocular motility for any disturbances or aberrant eyelid movements, and conducting a pupillary examination to identify signs of Horner syndrome or third cranial nerve palsy . Examination for giant papillary conjunctivitis or symblepharon is also performed . Corneal sensation and dry eye evaluation are critical due to the risk of postoperative keratopathy, a potential complication of ptosis surgery . Finally, a fundus examination is conducted to look for features of retinal pigmentary degeneration .
Specific eyelid measurements are meticulously taken with the patient’s face in the frontal plane, ensuring the frontalis muscle action is negated by gently holding the brow with the thumb, and with the eyes in the primary position of gaze . The examiner must be seated at the patient’s eye level to avoid parallax error and ensure accuracy .
Key measurements include:
- Palpebral Fissure Height (PFH): This is the vertical height of the palpebral aperture measured between the upper and lower eyelid margins in the pupillary plane. Normal PFH ranges from 7-10 mm in males and 8-12 mm in females, with an average of approximately 10 mm .
- Marginal Reflex Distance 1 (MRD 1): This measurement quantifies the vertical distance between the upper lid margin and the corneal light reflex. A normal MRD 1 is typically 4-5 mm . A decrease in this measurement directly indicates the presence and severity of ptosis. The difference in MRD 1 between the two eyes helps classify unilateral ptosis severity: a 2 mm difference indicates mild ptosis, 3 mm indicates moderate ptosis, and 4 mm or more indicates severe ptosis . A negative MRD 1 value signifies that the upper eyelid covers the corneal light reflex .
- Marginal Reflex Distance 2 (MRD 2): This is the distance between the corneal light reflex and the lower eyelid margin. Under normal conditions, the sum of MRD 1 and MRD 2 equals the PFH .
- Levator Action (Levator Function): This is the most crucial measurement for surgical planning. It assesses the amount of excursion of the upper eyelid when it moves from extreme downgaze to extreme upgaze, with the frontalis muscle’s action negated. Normal levator action is typically greater than 15 mm . The grading of levator action is critical for determining the choice of surgical procedure: less than 4 mm indicates poor function, 5 to 9 mm is fair, 9 to 11 mm is good, and greater than 12 mm is excellent .
- Margin Crease Distance (MCD): This measures the distance between the lid margin and the skin crease of the upper lid in downgaze. Normal values are 7-8 mm in men and 8-10 mm in women . An absent or vague lid crease is characteristic of congenital ptosis, while a higher than normal MCD is often observed in aponeurotic ptosis .
The detailed and specific eyelid measurements, including PFH, MRD1, MRD2, Levator Action, and MCD, are not merely descriptive but are essential for objectively determining the normal eyelid position and are crucial for surgical planning . The emphasis on “Levator Action” as the single most important measurement stems from its direct influence on the choice of surgical procedure . This highlights a highly quantitative and systematic approach to surgical decision-making in ptosis, where precise measurements directly translate into specific surgical strategies. For instance, poor levator action often dictates the need for a frontalis sling procedure. This methodical precision in assessment is fundamental to optimizing surgical outcomes.
Pharmacological Tests
Pharmacological tests are integral to the diagnostic process, providing targeted insights into specific etiologies of ptosis.
- Phenylephrine Test: This test is used to assess the function of Müller’s muscle and to predict the success of a Müller’s muscle-conjunctival resection (MMCR) surgery . Topical phenylephrine (typically 2.5% or 10% alpha-adrenergic sympathomimetic) is instilled into the superior fornix. Eyelid elevation in response to the drops indicates ideal candidacy for MMCR . An improvement in ptosis after phenylephrine administration also suggests a sympathetic etiology, such as Horner syndrome .
- Ice Test: This simple, non-invasive test has largely superseded the edrophonium test due to its safety and ease of administration . An ice pack is placed over the closed ptotic eyelid for 2 to 5 minutes . An improvement in Palpebral Fissure Height (PFH) by 2 mm or more after cooling is considered a positive result, strongly suggesting Myasthenia Gravis, as cooling improves neuromuscular transmission .
- Edrophonium Test (Tensilon Test): Historically used to assess Myasthenia Gravis . It involves the administration of edrophonium, a short-acting acetylcholinesterase inhibitor . A positive test is indicated by eyelid elevation within 2 to 5 minutes after administration . However, its relatively low sensitivity has led to its decreased use in favor of the ice test .
- Fatigue Test: In this test, the patient maintains fixation in upgaze for 30 seconds. In patients with Myasthenia Gravis, the eyelid will gradually drop due to muscle fatigue, demonstrating the characteristic fatigability of the condition .
The application of pharmacological tests like phenylephrine, ice, and edrophonium is highly specific, as each targets different underlying pathophysiologies, such as sympathetic innervation or neuromuscular junction dysfunction . This approach demonstrates a sophisticated diagnostic strategy that utilizes specific physiological responses to chemical or thermal stimuli to narrow down the etiology of ptosis, rather than relying solely on anatomical measurements. The widespread adoption of the ice test over the edrophonium test further exemplifies an evolution towards safer and equally effective diagnostic tools in clinical practice. Therefore, pharmacological tests are integral diagnostic tools in ptosis, providing targeted insights into specific etiologies by eliciting characteristic physiological responses, thereby guiding precise treatment planning.
Ancillary Investigations
Beyond clinical examination and pharmacological tests, several ancillary investigations are often necessary to establish a definitive diagnosis and identify any underlying systemic conditions.
- Antibody Testing: If Myasthenia Gravis is suspected, blood serum is tested for acetylcholine receptor antibodies, which are positive in approximately 50% of patients with solely ocular Myasthenia Gravis. Antistriated muscle antibodies and muscle-specific tyrosine kinase levels are also checked when Myasthenia Gravis is highly suspected . Given the known correlation between Myasthenia Gravis and thyroid disorders, thyroid function tests should also be assessed if thyroid involvement is suspected .
- Imaging Studies: X-rays, Computed Tomography (CT) scans, or Magnetic Resonance Imaging (MRI) scans of the brain and orbit are indicated when a pathology, such as a tumor in the orbit or skull, nerve defects, multiple sclerosis, or trauma, is suspected in these regions . Thorax radiography is used to assess the thymus gland in cases of Myasthenia Gravis, as thymic abnormalities are common in this autoimmune disorder . Imaging also provides valuable anatomical information crucial for surgical planning, particularly in complex cases .
- Genetic Testing: This may be considered if a syndromic condition, such as blepharophimosis, myotonic dystrophy, or oculopharyngeal muscular dystrophy (OPMD), is suspected as the cause of ptosis .
- Hertel Exophthalmometry: This measurement tool helps to rule out proptosis (forward displacement of the eyeball) or enophthalmos (backward displacement of the eyeball), thereby aiding in the exclusion of pseudoptosis .
The necessity for a range of investigations, including antibody testing, advanced imaging studies (CT, MRI, thorax radiography), and genetic testing, indicates that ptosis diagnosis often extends beyond the purview of a single ophthalmologist. This often requires collaboration with neurologists, geneticists, and other specialists. This highlights that ptosis can be a symptom of complex systemic diseases, necessitating a multidisciplinary approach for definitive diagnosis and comprehensive patient care.
Table 2: Standard Eyelid Measurements in Ptosis Diagnosis
| Measurement | Definition | Normal Range | Significance in Ptosis |
| Palpebral Fissure Height (PFH) | Vertical distance between upper and lower eyelid margins at pupillary plane | 7-10 mm (males), 8-12 mm (females); avg. 10 mm | Narrows in ptosis; used to quantify unilateral ptosis severity (mild 1-2mm, moderate 3-4mm, severe >4mm difference) |
| Marginal Reflex Distance 1 (MRD 1) | Vertical distance from corneal light reflex to central upper lid margin | 4-5 mm | Decreased in ptosis; difference between eyes classifies unilateral ptosis severity (2mm mild, 3mm moderate, 4mm severe) |
| Marginal Reflex Distance 2 (MRD 2) | Vertical distance from corneal light reflex to lower eyelid margin | ~5 mm | Used in conjunction with MRD1 to determine PFH (MRD1 + MRD2 = PFH) |
| Levator Action (LF) | Excursion of upper eyelid from extreme downgaze to extreme upgaze (frontalis negated) | >15 mm (Normal) | Crucial for surgical planning; graded as Poor (<4mm), Fair (5-9mm), Good (9-11mm), Excellent (>12mm) |
| Margin Crease Distance (MCD) | Distance between lid margin and skin crease in downgaze | 7-8 mm (men), 8-10 mm (women) | Absent/faint in congenital ptosis; higher than normal in aponeurotic ptosis |
This table standardizes the objective measurements utilized in ptosis diagnosis, providing clear parameters for consistent assessment and precise surgical planning. By defining normal ranges and illustrating how deviations indicate severity (e.g., MRD1 differences for unilateral ptosis), the table enables an objective grading of the condition, moving beyond subjective descriptions. The explicit connection between Levator Action and the choice of surgical procedure transforms this table into a direct clinical decision-making tool, translating diagnostic findings into actionable treatment pathways. These standardized measurements also serve as crucial benchmarks for monitoring disease progression over time or evaluating the efficacy of interventions, providing a quantitative basis for long-term follow-up care.
V. Management of Ptosis
The approach to managing ptosis is highly individualized, with treatment decisions contingent upon the underlying cause, the severity of the drooping, its impact on vision, and the functional integrity of the levator muscle . While mild cases of ptosis may not necessitate intervention, severe ptosis often requires treatment to prevent significant complications .
Non-Surgical Treatment Options
Non-surgical approaches generally offer a less definitive correction compared to surgery but can provide valuable temporary relief, particularly for mild cases or when surgical intervention is not feasible or desired . These methods are often favored for their lower risk profiles, minimal recovery times, and cost-effectiveness .
- Prescription Eye Drops:
- Oxymetazoline Hydrochloride 0.1% (Upneeq®): This is the only FDA-approved prescription eye drop specifically for acquired blepharoptosis . It functions as an alpha-adrenergic receptor agonist, selectively stimulating Müller’s muscle to induce an eyelid lift within minutes, with effects typically lasting approximately 6-8 hours . It is particularly indicated for involutional ptosis . Common adverse effects include punctate keratitis, conjunctival hyperemia (redness), dry eye, blurred vision, and headache . Prolonged use may lead to rebound redness .
- Other Adrenergic Agonists: Research has explored other agents like phenylephrine, cocaine, hydroxyamphetamine, apraclonidine, and naphazoline. While these have shown some short-term improvement in ptosis, their utility for therapeutic use is often limited by side effects or reduced long-term efficacy, making them more suitable for diagnostic purposes or occasional relief .
- Ptosis Crutches: These are small wire or plastic bars that can be attached to eyeglasses frames to apply gentle pressure to the eyelid, keeping it mildly elevated . Their use is less common in contemporary practice .
- Botox Injections: Botulinum toxin injections can be employed to address mild forms of eyelid ptosis or brow ptosis. By strategically relaxing depressor muscles, such as the orbicularis oculi, Botox allows the unopposed action of the frontalis muscle to lift the brows, thereby opening the eye area and improving overall symmetry . The effects typically last for 3-6 months .
- Dermal Fillers: Injected into key areas like the temples, lateral brow, and upper cheek, dermal fillers restore lost volume, creating a lifting effect that elevates the brow and opens the eyes . This approach is particularly effective when ptosis is associated with age-related volume depletion. Effects can persist for 6-24 months, depending on the type of filler used .
- Thread Lifting: This procedure involves inserting dissolvable threads beneath the skin to physically lift the brow and stimulate collagen production . It is best suited for patients with mild ptosis, providing immediate elevation and gradual skin tightening over several months .
- Energy-based Treatments (Ultrasound/Radiofrequency/Laser): These technologies utilize heat to stimulate collagen production in the deeper skin layers, resulting in gradual lifting and tightening of the skin in the brow and upper eyelid area . They are effective for mild to moderate ptosis primarily caused by loss of skin elasticity .
- Eyelid Exercises: While scientific studies have not definitively confirmed their efficacy as a standalone cure for ptosis, exercises such as blinking, squinting, and resistance training may help strengthen eyelid muscles and improve circulation .
The emergence and FDA approval of oxymetazoline eye drops, alongside the increasing utilization of cosmetic procedures like Botox, dermal fillers, and energy-based treatments, represent a notable shift towards non-surgical, less invasive options for managing ptosis . While these interventions often provide temporary or symptomatic relief rather than a definitive correction of underlying structural issues, their growing popularity reflects a demand for quick, low-risk aesthetic improvements and functional temporary lifts, especially for mild to moderate cases. This expansion of the therapeutic armamentarium beyond traditional surgery offers greater accessibility and patient choice in managing the condition.
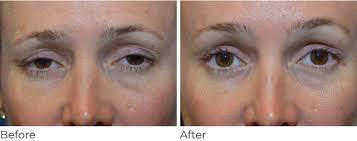
Surgical Interventions
Surgery remains the primary and often most effective treatment for ptosis, particularly in severe cases or when vision is significantly impaired . The selection of the appropriate surgical technique is highly individualized, depending critically on the underlying cause of ptosis and, most importantly, the functional capacity of the levator muscle .
- Levator Advancement (External Approach):
- Indications: This is the most commonly performed surgical procedure for ptosis and is recommended for patients who have good levator muscle function .
- Procedure: An incision is typically made in the natural eyelid crease to minimize visible scarring . The surgeon then repositions the attachment of the levator muscle by stitching it to the tarsus (the connective tissue within the eyelid) or shortens the levator muscle by a prescribed amount . This procedure is often performed under local anesthesia with sedation, allowing the patient to cooperate with eye movements for intraoperative adjustments, which helps the surgeon achieve the optimal eyelid height and contour .
- Outcome: The primary goal is an elevated eyelid that improves both vision and cosmetic appearance .
- Müller’s Muscle-Conjunctival Resection (MMCR) (Internal Approach):
- Indications: This technique is suitable for mild to moderate ptosis in patients with good levator function, especially those who demonstrate a positive response to the phenylephrine test, and who do not have significant excess upper eyelid skin .
- Procedure: The eyelid is everted, and a measured portion of Müller’s muscle (and an overlying section of conjunctiva) is shortened from the inside of the eyelid . A key advantage of this internal approach is that it leaves no external scar .
- Outcome: MMCR offers predictable outcomes, helps preserve the natural lid contour, and is associated with a relatively quick recovery period . Success rates have been reported between 90-97% for appropriately selected candidates .
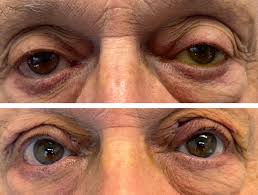
- Frontalis Sling Fixation:
- Indications: This is the preferred procedure for patients with poor levator action (typically less than 4 mm) . It is commonly employed for severe congenital ptosis, as well as ptosis secondary to conditions such as chronic progressive external ophthalmoplegia (CPEO), third cranial nerve palsy, blepharophimosis syndrome, and Myasthenia Gravis .
- Procedure: The upper eyelid is directly attached to the frontalis muscle, located above the eyebrows, using a sling material (e.g., a small silicone rod, autologous fascia lata, or other synthetic materials) . This connection allows the stronger forehead muscles to elevate the eyelid . This procedure is typically performed under general anesthesia .
- Outcome: This technique is highly effective in cases where the levator muscle function is very weak or absent.
The consistent emphasis across various sources that the “Levator Action” measurement is the single most important factor determining surgical choice highlights a fundamental principle in ptosis management: treatment is highly individualized and directly dictated by the functional capacity of the primary eyelid elevator muscle . This establishes a clear decision-making algorithm: patients with good levator function are typically candidates for levator advancement or Müller’s muscle-conjunctival resection, while those with poor function necessitate a frontalis sling. This systematic approach optimizes surgical outcomes by precisely matching the intervention to the underlying pathophysiology, ensuring the most effective and appropriate treatment for each patient.
Table 3: Comparison of Surgical Approaches for Ptosis
| Surgical Approach | Primary Indication | Levator Function | Advantages | Disadvantages/Considerations |
| Levator Advancement (External Approach) | Most common for ptosis correction | Good | Effective, adjustable, hidden scar in eyelid crease | Requires patient cooperation (local anesthesia) |
| Müller’s Muscle-Conjunctival Resection (MMCR) (Internal Approach) | Mild to moderate ptosis, positive phenylephrine test | Good | No external scar, predictable outcome, quick recovery | Not suitable for significant excess skin or poor levator function |
| Frontalis Sling Fixation | Severe ptosis, poor levator muscle function | Poor (<4 mm) | Utilizes stronger forehead muscles to lift eyelid | Common side effect: temporary inability to completely close eye (lagophthalmos), often requires general anesthesia |
This table provides a clear, comparative overview of the main surgical techniques for ptosis, directly linking them to their indications and functional outcomes. By presenting the three primary surgical approaches side-by-side, it allows for easy comparison of their mechanisms and suitability for different patient profiles, which is essential for clinicians to make informed decisions. The explicit connection between “Levator Function” and the choice of procedure directly supports clinical decision-making. This resource can also be valuable for patient education, helping individuals understand why a particular surgical approach is recommended for their specific type of ptosis, including the associated benefits and potential drawbacks.
VI. Complications and Prognosis
Complications of Untreated Ptosis
If left untreated, particularly in children, ptosis can lead to significant and potentially irreversible long-term complications .
- Amblyopia (“Lazy Eye”): This is one of the most severe complications, especially in pediatric patients. It occurs when the drooping eyelid physically obstructs the visual axis, causing the brain to suppress the visual input from the affected eye and favor the eye with clearer vision . If not addressed during the critical period of visual development (typically by age six), this can lead to permanent vision loss in the affected eye .
- Astigmatism: The constant pressure exerted by the drooping eyelid on the developing cornea can alter its shape, leading to astigmatism, which results in distorted or wavy vision .
- Compensatory Head and Neck Deformities: Children with ptosis often adopt a “chin-up” position or continuously raise their eyebrows to see beneath the drooping eyelid . Over many years, these abnormal head positions can lead to chronic neck problems, tightened forehead muscles, and even deformities in the head and neck .
- Psychosocial Impact: Beyond the physical and visual consequences, ptosis can significantly affect a patient’s self-perception and quality of life. It can lead to cosmetic complaints, feelings of tiredness, or an appearance of inattentiveness, which can have psychosocial implications .
The repeated emphasis on amblyopia as a severe complication, particularly in children, and the explicit statement that “early surgical intervention is often recommended to prevent these complications and to ensure normal visual development” underscore a critical temporal window for treatment. This implies that for pediatric ptosis, the functional imperative of preventing permanent vision loss overrides cosmetic considerations, making it a more urgent medical issue than in adults, where the primary concerns might be quality of life and appearance. Therefore, untreated ptosis in children is a time-sensitive medical concern due to the irreversible risk of amblyopia and associated developmental issues, necessitating prompt intervention to preserve visual potential.
Post-Surgical Complications (Early and Late)
Despite meticulous planning and execution, complications can arise following ptosis repair surgery .
- Early Complications:
- Asymmetry: Postoperative asymmetry in eyelid height and shape is the most common early complication . While often present immediately after surgery, it may resolve spontaneously with time as swelling subsides .
- Undercorrection or Overcorrection: These are common occurrences postoperatively and may also resolve over time . Mild overcorrection can sometimes be managed conservatively with eyelid traction exercises, where the patient gently pulls on the eyelashes while looking downward . However, severe overcorrection typically necessitates immediate revision surgery .
- Surgical Site Infection and Bleeding/Hematoma: These are acute but uncommon complications .
- Exposure Keratopathy: This is a significant concern requiring proper monitoring . Postoperative mild lagophthalmos (incomplete eyelid closure) and a decreased blink rate are common, contributing to ocular dryness, but usually resolve within 2 to 3 weeks .
- Late Complications:
- Persistent Lagophthalmos and Exposure Keratopathy: These can become chronic complications of ptosis repair surgery, requiring ongoing management with lubricating eye drops and ointments, or in severe cases, revision surgery to lower the lid height .
- Recurrent Ptosis: This is a chronic complication that often necessitates revision surgery . Recurrence rates vary depending on the surgical technique and material used. For aponeurotic ptosis, studies show recurrence rates ranging from 12% to 39% eight to ten years after the procedure . For frontalis sling procedures, recurrence is reported to be around 26% after 20 months . Myogenic ptosis, being a progressive disorder, has a particularly high rate of recurrence despite surgical interventions .
- Eyelid Contour Defects: These can result from improper placement of sutures on the tarsus. Small contour defects may improve over time with lid massage, but persistent or severe abnormalities require revision surgery to ensure proper suture placement .
- Conjunctival Prolapse: Can occur due to extensive dissection between the conjunctiva and levator, disrupting suspensory ligaments. Mild prolapse can be treated conservatively with lubricants, while severe cases may require repositioning with sutures .
- Eyelash Ptosis: This can occur due to excessive dissection of the orbicularis muscle from the tarsus and can be corrected by anterior lamella repositioning sutures .
- Suture Granuloma: Small inflammatory nodules can form at the suture site and may require excision .
The frequent mention of lagophthalmos and exposure keratopathy as complications, particularly after frontalis sling surgery, highlights a critical trade-off inherent in ptosis surgery . While the primary objective is to lift the eyelid, overcorrection or an inability to completely close the eye can severely compromise corneal health, leading to dryness, irritation, and potential vision impairment. This necessitates careful preoperative assessment, including evaluation of Bell’s phenomenon and tear film quality, and often leads to a “planned under-correction” in high-risk patients to prioritize ocular surface integrity over perfect cosmetic symmetry. Therefore, ptosis surgery involves a delicate balance between achieving optimal eyelid elevation and preserving ocular surface health, with potential complications like lagophthalmos and exposure keratopathy necessitating careful patient selection, meticulous surgical technique, and vigilant postoperative management.
Prognosis and Recurrence Rates by Ptosis Type
The expected long-term outcome and likelihood of recurrence in ptosis are highly dependent on its underlying etiology .
- Congenital Ptosis: Surgical correction can yield excellent functional and cosmetic results . With careful observation and appropriate treatment, amblyopia, if present, can be successfully managed . However, it is important to note that 50% or more of patients with congenital ptosis may require repeat surgery within 8-10 years following the initial procedure . Early diagnosis and treatment are crucial to prevent the development of amblyopia and subsequent permanent vision loss .
- Aponeurotic Ptosis (Involutional): Surgery for aponeurotic ptosis generally has a high success rate . Nevertheless, ptosis can naturally reoccur over time due to the ongoing aging processes that continue to stretch and thin the levator aponeurosis. Recurrence rates for this type range from 12% to 39% eight to ten years after the initial procedure .
- Neurogenic Ptosis: The prognosis for neurogenic ptosis varies significantly depending on the specific underlying neurological cause. For instance, pupil-sparing third nerve palsy resulting from ischemic causes often resolves spontaneously within three months . Treatment for Horner syndrome primarily focuses on addressing the underlying systemic cause . Surgical treatment for neurogenic ptosis can be challenging, especially in cases with a poor Bell’s phenomenon, due to the increased risk of exposure keratopathy .
- Myogenic Ptosis: Myogenic ptosis is recognized as a progressive disorder with a high rate of recurrence, even after appropriate surgical interventions . While surgery may improve the appearance of the eyelid and partially restore function, the underlying muscle weakness tends to persist and recur over time .
- Traumatic Ptosis: Ptosis secondary to blunt trauma may resolve spontaneously over time. Surgical repair is typically considered if there is no significant improvement after 6 months . The overall prognosis for traumatic ptosis depends on the specific mechanism of injury and the extent of damage to the eyelid structures .
The varying prognoses and recurrence rates across different ptosis types—such as the high recurrence in myogenic ptosis, the natural recurrence in aponeurotic ptosis due to ongoing aging, and the potential for spontaneous resolution in some traumatic or neurogenic cases—highlight that ptosis is often a chronic or recurring condition rather than a one-time fix . This necessitates that patient counseling includes realistic expectations about long-term outcomes, the potential need for revision surgeries, and the importance of ongoing monitoring, particularly for progressive conditions like myogenic ptosis. This understanding shifts the clinical focus from a singular “cure” to a comprehensive, long-term “management” strategy for many types of ptosis.
Table 4: Prognosis and Recurrence Rates by Ptosis Type
| Ptosis Type | General Prognosis | Recurrence Rate/Considerations |
| Congenital | Excellent functional & cosmetic results with surgery; amblyopia treatable | >50% may require repeat surgery in 8-10 years |
| Aponeurotic (Involutional) | High surgical success rate | Can naturally reoccur (12-39% in 8-10 years) due to ongoing aging |
| Neurogenic | Varies widely by underlying cause; some (e.g., ischemic CN III palsy) may resolve spontaneously | Dependent on underlying neurological condition; surgical treatment challenging with poor Bell’s phenomenon |
| Myogenic | Progressive disorder; surgical improvement in appearance is possible | High rate of recurrence despite surgical interventions |
| Traumatic | May resolve spontaneously (blunt trauma); surgical repair if no improvement after 6 months | Varies by specific injury mechanism and extent of damage |
This table synthesizes crucial information about the long-term outlook and potential for recurrence across different ptosis types, which is essential for effective patient counseling and setting realistic expectations. For patients considering surgery, understanding the likelihood of recurrence or the progressive nature of their condition is vital. This table provides concrete data points that clinicians can use to discuss long-term management plans. The prognosis also influences the aggressiveness of treatment and the frequency of follow-up. For example, recognizing the high recurrence rate in myogenic ptosis may lead to considering different surgical strategies or the use of ptosis crutches that account for disease progression. This comprehensive view reinforces that ptosis is often a manageable condition requiring ongoing care and potentially multiple interventions throughout a patient’s lifetime, especially for certain etiologies.
VII. Recent Advancements in Ptosis Understanding, Diagnosis, and Treatment
The field of oculoplastic surgery is in a state of continuous evolution, marked by significant advancements in diagnostic tools and treatment modalities for ptosis. These innovations are reshaping how ptosis is understood, detected, and managed, offering more precise and effective solutions.
Novel Diagnostic Tools
Recent advancements in Artificial Intelligence (AI) offer promising solutions for automated and precise ptosis measurement, potentially revolutionizing clinical assessment .
- AI-based Measurement: AI models, often built upon neural network architectures such as ResNet50, are capable of automating the measurement of key parameters like Margin Reflex Distance 1 (MRD1) and Palpebral Fissure Height (PFH) . These models can process data from various sources, including standard patient photographs and even “selfie” video clips recorded on smartphones . Some advanced systems also provide analysis of the Corneal Exposure Rate (CER) .
- Advantages: The benefits of AI-based tools include enhanced precision, accuracy, and consistency compared to traditional manual measurements, which are prone to human variability . These tools provide faster, almost instantaneous results, significantly reducing diagnostic time and potential human error . Furthermore, the ability to collect data remotely via smartphones is particularly beneficial for monitoring disease progression in conditions like Myasthenia Gravis, allowing for more frequent and convenient assessments without requiring constant clinic visits .
The development of AI-based tools for ptosis measurement, particularly their capability to analyze selfie video clips recorded on smartphones, represents a significant step towards democratizing access to precise diagnostics . This technology could enable more frequent and convenient monitoring of ptosis, especially for patients in remote areas or those with mobility challenges, potentially leading to earlier detection of subtle changes and more timely interventions. It also signals a shift in the diagnostic paradigm from solely in-clinic measurements to continuous, patient-generated data, which holds the potential to revolutionize long-term disease management and research by providing a richer, more dynamic dataset.
Emerging Non-Surgical Therapies
While surgical intervention remains the gold standard for definitive correction of ptosis, topical pharmaceuticals are gaining traction as viable options for short-term relief .
- Oxymetazoline Hydrochloride 0.1% (Upneeq®): This FDA-approved eye drop represents a significant advancement in non-surgical ptosis management . It works by selectively stimulating alpha-adrenergic receptors in Müller’s muscle, leading to temporary eyelid elevation . Clinical trials have demonstrated its efficacy, showing significant improvement in eyelid elevation and the superior visual field within minutes of administration, with effects lasting approximately 6-8 hours . It offers a non-surgical option primarily for acquired blepharoptosis, particularly involutional ptosis .
- Other Adrenergic Agonists: Research has also explored other agents such as phenylephrine, cocaine, hydroxyamphetamine, apraclonidine, and naphazoline. While these compounds have shown some capacity for short-term improvement in ptosis, their therapeutic use is often limited by a short duration of action or notable systemic and ocular side effects, making them more suitable for diagnostic purposes or occasional, temporary relief .
The specific FDA approval of oxymetazoline for acquired blepharoptosis, alongside ongoing research into other adrenergic agonists, indicates a targeted pharmacological approach to ptosis that leverages the sympathetic innervation of Müller’s muscle . This development signals a growing recognition of a “pharmacological niche” for mild to moderate cases, where patients may prefer non-invasive, temporary solutions over surgery for cosmetic or functional reasons, or as a temporary measure while awaiting surgical intervention. This represents a significant expansion of the therapeutic options available, broadening treatment accessibility and patient choice.
Innovations in Surgical Techniques
Surgical interventions for ptosis continue to evolve, with a strong emphasis on enhancing precision, minimizing invasiveness, and improving operational efficiency.
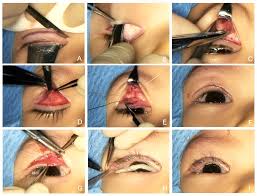
- Adjustable Suture Techniques: These techniques have significantly advanced ptosis repair by enabling surgeons to fine-tune the eyelid position post-operatively . This capability enhances precision and accuracy, reducing the risk of common complications such as undercorrection or overcorrection, and ultimately leading to improved patient outcomes and satisfaction .
- Minimally Invasive Approaches: Driven by the desire for reduced recovery times and scarring, these techniques involve smaller incisions and less tissue disruption . This results in faster healing, reduced postoperative discomfort, and improved cosmetic outcomes, aligning with patient preferences for less invasive procedures .
- Assistant-Free Techniques: Novel surgical methods have been developed, such as combining small fish hook retractors with frilled disposable drapes . This innovative approach allows surgeons to perform levator aponeurosis advancement independently, providing stable visualization of the surgical field and ease of adjustment without requiring an assistant . This technique has demonstrated favorable results in a large series of eyelids, particularly useful in settings with limited access to surgical assistants .
The collective focus on adjustable suture techniques, minimally invasive approaches, and assistant-free techniques underscores a strong trend in surgical innovation towards greater precision, reduced invasiveness, and improved operational efficiency . Adjustable sutures directly address common complications like under- or overcorrection, while minimally invasive methods cater to patient demands for faster recovery and superior cosmetic results. Assistant-free techniques, though seemingly niche, can enhance the accessibility and standardization of care, particularly in resource-constrained environments. These advancements collectively reflect a maturing surgical field that continually strives for optimized patient experience and outcomes.
Genetic Research Insights
Genetic research is increasingly contributing to a deeper understanding of the molecular underpinnings of ptosis, particularly congenital forms.
- Congenital Ptosis Genetics: While often idiopathic, congenital ptosis can occur through autosomal dominant inheritance, with familial occurrences strongly suggesting underlying genetic or chromosomal defects . Histological studies of the levator muscles in congenital ptosis patients reveal a dystrophic condition, where normal muscle tissue is infiltrated or replaced by fat and fibrous tissue, indicating localized developmental defects in muscle structure .
- Specific Genetic Subtypes: Researchers have identified several genetic subtypes of ptosis, including those associated with Horner syndrome, blepharophimosis, Steinert Disease, and Congenital Fibrosis .
- Gene Research: Ongoing research aims to elucidate the role of specific genes, such as ZFHX4/ZFH-4, and to understand how mutations in these genes manifest the ptosis effect .
The growing body of genetic research, including studies on inherited forms and specific genetic syndromes like blepharophimosis, alongside efforts to identify genes such as ZFHX4, indicates a profound scientific inquiry into the molecular and genetic underpinnings of ptosis . This shift from purely anatomical or clinical descriptions to genetic mechanisms suggests a future where ptosis diagnosis might routinely involve genetic screening, and treatments could potentially become gene-targeted, offering more fundamental insights and potentially curative approaches for inherited forms of the condition.
VIII. Conclusion
Ptosis, or blepharoptosis, is a multifaceted condition characterized by the drooping of the upper eyelid, which can significantly impair both visual function and cosmetic appearance. Its diverse etiologies span congenital maldevelopment of the levator muscle to acquired forms resulting from aging, neurological disorders, primary muscle diseases, mechanical obstructions, and traumatic injuries.
Accurate diagnosis is paramount and relies on a comprehensive patient history, a meticulous clinical examination that includes precise eyelid measurements such as Palpebral Fissure Height (PFH), Marginal Reflex Distance 1 (MRD1), and Levator Action, and targeted pharmacological tests like the Phenylephrine and Ice tests to differentiate underlying causes. Ancillary investigations, including antibody testing and various imaging modalities, are crucial for identifying systemic associations and guiding further management.
Management strategies are highly individualized, adapting to the specific etiology and severity of the ptosis. Non-surgical options, such as the FDA-approved oxymetazoline eye drops, offer temporary relief for mild to moderate cases, representing a significant advancement in symptomatic management. For definitive correction, various surgical interventions are available, including levator advancement, Müller’s muscle-conjunctival resection, and frontalis sling fixation. The choice of surgical technique is critically determined by the patient’s levator muscle function, underscoring the precise link between diagnostic assessment and therapeutic strategy.
Untreated ptosis, particularly in children, carries substantial risks, including irreversible amblyopia, astigmatism, and musculoskeletal deformities from compensatory head posturing. Post-surgical complications, such as asymmetry, undercorrection, overcorrection, lagophthalmos, exposure keratopathy, and recurrence, necessitate careful preoperative assessment, realistic patient counseling, and diligent long-term follow-up. The prognosis and likelihood of recurrence are highly dependent on the specific type of ptosis, with conditions like myogenic ptosis often being progressive and prone to recurrence despite intervention.
Recent advancements are transforming the field, with Artificial Intelligence-based tools promising enhanced precision and accessibility in diagnosis, enabling remote monitoring. Innovations in surgical techniques, including adjustable sutures, minimally invasive approaches, and assistant-free procedures, aim to improve precision, efficiency, and patient outcomes. Concurrently, ongoing genetic research is deepening the understanding of inherited forms of ptosis at a molecular level, paving the way for potential future gene-targeted therapies. A multidisciplinary approach, integrating insights from ophthalmology, neurology, and genetics, remains essential for comprehensive patient care, ensuring both optimal functional improvement and aesthetic satisfaction.
References
- Wong JF, White P, Al-Abri R. Ptosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546705/
- Patel P, Mettu P, Lee JJ. Ptosis Correction. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539828/
- Hussain I, Moshirfar M, Mian SI. Anatomy, Head and Neck: Levator Palpebrae Superioris Muscle. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536921/
- Eyeplastics. Ptosis (Droopy Eyelid) Lazy Eyelid Levator Muscle Mullers Muscle [Internet]. [cited 2024 May 29]. Available from: https://www.eyeplastics.com/ptosis-droopy-eyelid-lazy-eyelid-levator-muscle-mullers-muscle.html
- Lee JJ, Patel P, Mettu P. Congenital Ptosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK568688/
- JOMI. Internal Ptosis Repair by Mullers Muscle Resection [Internet]. [cited 2024 May 29]. Available from: https://jomi.com/article/514/internal-ptosis-repair-by-mullers-muscle-resection
- See Vividly. Ptosis (Droopy Eyelid) [Internet]. [cited 2024 May 29]. Available from: https://www.seevividly.com/info/Eye_Problems/Ptosis
- Mount Sinai. Eyelid drooping [Internet]. [cited 2024 May 29]. Available from: https://www.mountsinai.org/health-library/diseases-conditions/eyelid-drooping
- NCBI. Ptosis – StatPearls – NCBI Bookshelf [Internet]. [cited 2024 May 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546705/#:~:text=Ptosis%20is%20known%20as%20the,mechanical%2C%20or%20traumatic%20in%20origin.
- NCBI. Ptosis – StatPearls – NCBI Bookshelf [Internet]. [cited 2024 May 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546705/
- Cleveland Clinic. Ptosis (Droopy Eyelid): Causes & Treatment [Internet]. [cited 2024 May 29]. Available from: https://my.clevelandclinic.org/health/diseases/14418-ptosis-droopy-eyelid
- Pocket Eye. How should I assess my patient’s eyelids? [Internet]. [cited 2024 May 29]. Available from: https://pocket-eye.com/blog/f/how-should-i-assess-my-patient%E2%80%99s-eyelids?blogcategory=News
- Optometry Times. How to identify ptosis in clinical practice [Internet]. [cited 2024 May 29]. Available from: https://www.optometrytimes.com/view/how-to-identify-ptosis-in-clinical-practice
- Prasad Cosmetic Surgery. Eyelid Ptosis Causes – Possible Neurological Causes [Internet]. [cited 2024 May 29]. Available from: https://prasadcosmeticsurgery.com/eyelid-ptosis-causes-possible-neurological-causes/
- Espaillat Cabral. What is neurogenic ptosis? [Internet]. [cited 2024 May 29]. Available from: https://www.espaillatcabral.com/en/blog/item/que-es-la-ptosis-neurogenica
- EyeWiki. Myogenic Ptosis [Internet]. [cited 2024 May 29]. Available from: https://eyewiki.org/Myogenic_Ptosis
- Review of Optometry. The Upside of a Drooping Lid [Internet]. [cited 2024 May 29]. Available from: https://www.reviewofoptometry.com/article/the-upside-of-a-drooping-lid
- Myasthenia gravis news. Myasthenia gravis ice pack test: A step-by-step guide [Internet]. [cited 2024 May 29]. Available from: https://myastheniagravisnews.com/ice-pack-test-myasthenia-gravis/
- NCBI. Ptosis Correction – StatPearls – NCBI Bookshelf [Internet]. [cited 2024 May 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539828/
- IMO Grupo Miranza. Ptosis (drooping eyelids): causes and treatments [Internet]. [cited 2024 May 29]. Available from: https://www.imo.es/en/disorders/ptosis-drooping-eyelids-oculoplastic/
- PMC. Traumatic Ptosis: Evaluation of Etiology, Management and Prognosis [Internet]. [cited 2024 May 29]. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC6210876/
- Valerie Vick, MD. Ptosis [Internet]. [cited 2024 May 29]. Available from: https://valerievick.com/ptosis/
- EyeWiki. Frontalis Suspension Procedure [Internet]. [cited 2024 May 29]. Available from: https://eyewiki.org/Frontalis_Suspension_Procedure
- PMC. Frontalis suspension is a commonly used surgery that is indicated in patients with blepharoptosis and poor levator muscle function [Internet]. [cited 2024 May 29]. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3040462/
- RSIP Vision. Image Analysis Evaluating Blepharoptosis/Eyelid Drooping by RSIP Vision [Internet]. [cited 2024 May 29]. Available from: https://www.rsipvision.com/eyelid-drooping-blepharoptosis/
- Eyes On Eyecare. The Ultimate Guide to Assessing Eyelid Ptosis [Internet]. [cited 2024 May 29]. Available from: https://eyesoneyecare.com/resources/the-ultimate-guide-to-assessing-eyelid-ptosis/
- PMC. Clinical value of phenylephrine testing in the upper and lower eyelids of patients with aponeurotic and congenital eyelid ptosis [Internet]. [cited 2024 May 29]. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC9453416/
- Review of Optometry. The Ophthalmic Workhorse [Internet]. [cited 2024 May 29]. Available from: https://www.reviewofoptometry.com/article/the-ophthalmic-workhorse
- Hubmeded. Ptosis Treatment Without Surgery: Effective Non-Surgical Options [Internet]. [cited 2024 May 29]. Available from: https://www.hubmeded.com/blog.