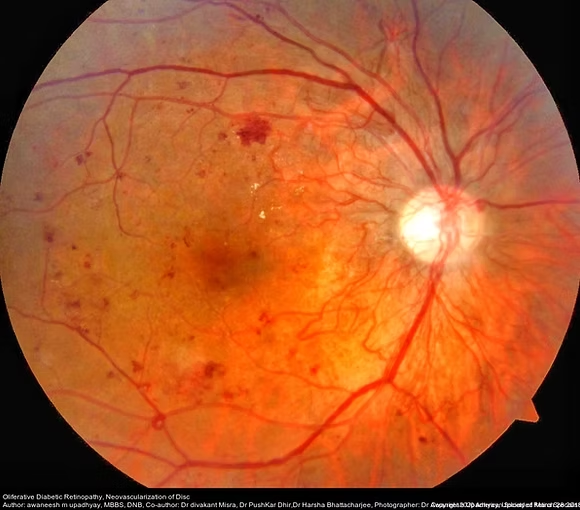
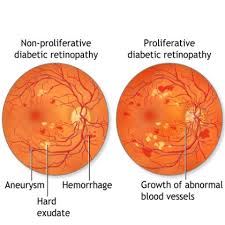
Diabetic retinopathy (DR) is a significant and growing public health concern, standing as a major micro vascular complication of diabetes and a leading cause of visual impairment and blindness globally, particularly in adults over 50. With the increasing number of individuals affected by diabetes worldwide, the prevalence of DR is also on the rise. DR progresses through stages, from non-proliferative (NPDR) to the more severe proliferative diabetic retinopathy (PDR), which is characterized by neovascularization. Another common complication, diabetic macular edema (DME), involves retinal leakage and edema, significantly impacting central vision. Early diagnosis and intervention are crucial to preventing vision loss.
The Role of Anti-VEGF Therapy
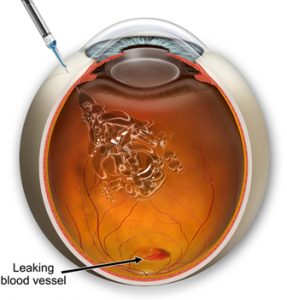
For many years, treatments for DR primarily involved retinal laser photocoagulation and vitrectomy for advanced stages. While these treatments are valuable, laser treatment can sometimes lead to visual field narrowing, and vitrectomy is typically reserved for advanced lesions. A significant advancement in managing DR, particularly its neovascular complications and macular edema, has been the introduction of intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections.
Retinal neovascularization, a hallmark of advanced DR, is driven by factors like hypoxia, ischemia, and inflammation, disrupting the balance of vascular endothelial growth factor (VEGF) and pigment epithelium-derived factor (PEDF). VEGF is a potent angiogenic factor that plays a central role in the development of DR. Anti-VEGF drugs work by inhibiting these proangiogenic factors, specifically targeting VEGF to counteract pathological neovascularization and disease progression. The aim is to stabilize vision and, in some cases, achieve recovery.
Anti-VEGFs variants
Anti-vascular endothelial growth factor (anti-VEGF) therapy has become a standard treatment modality for controlling angiogenesis and preventing further deterioration in diabetic retinopathy (DR). These agents are described as widely utilized therapeutic interventions for DR and are the main treatment for early and advanced DR. The purpose of anti-VEGF treatments is to counteract pathological neovascularization and disease progression, aiming to stabilize or even improve vision. Compared to conventional laser treatment, anti-VEGF therapy can improve vision with fewer ocular adverse effects.
Several specific anti-VEGF drugs used in the treatment of DR:
- Ranibizumab: This drug is a recombinant fragment of a humanized monoclonal antibody that targets VEGF. It targets all VEGF-A isoforms. Ranibizumab was approved by the United States Food and Drug Administration (FDA) in 2017 for the treatment of all stages of diabetic retinopathy. It has been used in studies for macular edema secondary to DR, including a generic formulation named Razumab. Ranibizumab is noted to have comparable therapeutic efficacy to aflibercept in prior research.
- Bevacizumab: Like ranibizumab, bevacizumab also targets all VEGF-A isoforms. It has been mentioned in the context of preoperative anti-angiogenic therapy for patients with proliferative diabetic retinopathy.
- Aflibercept: This drug is a recombinant fusion protein that blocks VEGF-A, VEGF-B, and placental growth factor. Aflibercept currently demonstrates significant efficacy in the management of diabetic retinopathy. One source notes that aflibercept may require fewer injection needles compared to ranibizumab, although a specific study mentioned found no statistically significant difference, possibly due to a limited sample size.
For patients with DR and diabetic macular edema (DME), anti-VEGF therapy is indicated when there is significant visual impairment, confirmed through diagnosis via patient history, visual acuity testing, fundus examination, and OCT imaging.
Advancements and Challenges
While anti-VEGF therapy is promising, it often requires repeated intraocular injections, which can be challenging to successfully implement and affects treatment efficacy and outcomes. Visual outcomes are dependent on injection frequency, making patient adherence crucial for successful therapy. Barriers to adherence are significant and can include visit frequency, travel logistics, waiting time, appointment access, caregiver support, and financial burden. Patient perception of treatment effectiveness and communication with their doctor also play a role.
Studies have investigated the effectiveness of anti-VEGF therapy in various contexts. For example, a study using ranibizumab for macular edema secondary to DR or retinal vein occlusion followed patients with monthly injections until resolution or up to three injections. While ranibizumab is a widely used anti-VEGF agent, generic formulations are also used, as seen in a study where Razumab was the hospital supply drug.
Research is also exploring factors that might influence the response to anti-VEGF therapy. For instance, studies suggest that patients with macular edema who also have obstructive sleep apnea syndrome (OSAS) may have a poorer response to anti-VEGF therapy, although more extensive research is needed in this area. A study evaluating ranibizumab in patients with DR and retinal vein occlusion found that while there was a significant improvement in visual acuity in patients with mild to moderate OSAS, those with severe OSAS showed only minimal, statistically insignificant improvement, suggesting that OSAS severity could influence therapeutic response. However, the study did not find a significant correlation between the number of injections needed and OSAS severity.
Limitations of anti-VEGF therapy mentioned in the sources include its high cost and invasive nature compared to non-invasive treatments. However, ongoing research aims to improve adherence and outcomes. This includes exploring new therapies offering convenience, long-acting effectiveness, and better tolerability. Improved doctor-patient communication and patient education are also seen as essential strategies to enhance treatment adherence.
In summary, anti-VEGF therapy has revolutionized the treatment of diabetic retinopathy, offering a direct way to combat pathological neovascularization and macular edema. While challenges like the need for repeated injections and ensuring patient adherence remain, ongoing research continues to refine treatment strategies and explore factors influencing outcomes, paving the way for more effective and accessible care for patients with DR.
References
- Abualhasan H, Beshtawi IM, Hantoli S, Noor M, Mustafa O. Predictive factors for adherence to intravitreal anti-vascular endothelial growth factor treatment injections. BMC Ophthalmol. 2025;25(268):2-8.
- Banerjee et al. Effect of Obstructive Sleep Apnea on the Severity of Diabetic Macular Edema, Treatment Response to Anti-VEGF Therapy, and Visual Outcome in Patients with Diabetic Retinopathy and Retinal Vein Occlusions. Cureus. 2025;17(3):e81385.
- Chen X, Li J, Guo Q, Xu N, Huang L, Miao H. The association between urinary caffeine metabolites and diabetic retinopathy in individuals with type 2 diabetes: a cross-sectional.
- Harley et al. Exploring leukocyte differential count ratios as potential biomarkers for diabetic retinopathy in type 2 diabetes mellitus patients: a systematic review. BMC Ophthalmol. 2025;25(265):1-20.
- Huang [Authors not explicitly listed in source excerpt]. Reduced risk of diabetic retinopathy among patients with osteoarthritis after joint replacement.
- Li J, Li Y, Qi Q, Chen N, Zhang Y. Predictive value of triglyceride glucose index and systemic inflammation index for diabetic retinopathy in Type-2 diabetes. Pak J Med Sci. 2025;41(4):1072-1077.
- Long et al. EfficientNetB0-Based End-to-End Diagnostic System for Diabetic Retinopathy and Its Complications Using Fluorescein Angiography.
- Lu et al. Analysis of risk factors for vitreous hemorrhage after vitrectomy in patients with diabetic retinopathy. BMC Ophthalmol. 2025;25(274):8-10.
- Raimondi R, Sow K, Peto T, Wride N, Habib MS, Sproule A, et al. The effect of intraocular pressure setting during phacoemulsification on macular and peripapillary microvasculature: a masked observer randomized controlled feasibility trial.
- Sushith [Authors not explicitly listed in source excerpt]. A hybrid deep learning framework for accurate detection and classification of diabetic retinopathy.
- Wang J, Su J, Ma J, Liu D. Causal roles of lipids and mediating biomarkers in the development of diabetic retinopathy: a Mendelian randomization study. Diabetol Metab Syndr. 2025;17(139):4-11.
- Xie L, Peng YQ, Shen X. Identifying therapeutic target genes for diabetic retinopathy using Mendelian randomization [Derived from filename and title header]. Diabetol Metab Syndr. 2025;17(145):14.
- Yang [Authors not explicitly listed in source excerpt]. Combining Network Pharmacological Analysis and Molecular Docking to Explore the Mechanism of ZYZC Formula in the Treatment of Diabetic Retinopathy [Derived from filename and content]. Diabetes Metab Syndr Obes. 2025.
- Yi G, Yu H. Risk factors for pupil changes in patients with diabetic retinopathy undergoing phacoemulsification cataract surgery. BMC Ophthalmol. 2025;25(252):3-8.
- Yin C, Li H. Diagnostic value of choroidal vascular density in different regions of diabetic retinopathy: a cross-sectional study.